RELATED Live.
- About us
- KIMA Members
-
KIMA Doctors
- All
- Anesthesiology
- artificial joint center
- Breast and Endocrine Surgery
- Breast cancer and thyroid cancer center
- Breast Surgery
- Cardiology
- Cardiothoracic Surgery
- Cerebrovascular Center
- Colorectal Surgery
- dental and maxillofacial surgery
- Dermatology
- Endocrinology
- Gastroenterology
- General Surgery
- Genito-Urology
- Hematology
- Hemato-oncology
- Infection Center
- Internal Medicine
- International Healthcare Center
- Korean Medicine
- liver center/Pancreas and billiary tract center
- Liver Transplantation
- Neurology
- Neurosurgery
- Obstetrics & Gynecology
- Ophthalmology
- Orthopedic
- Otorhinolaryngology
- Pediatric & Juvenile
- Pediatric Allergy and Respiratory Diseases
- Pediatric Gastroenterology
- Pediatric Neurology
- Pediatrics
- Physical Medicine & Rehabilitation
- Plastic & Reconstructive Surgery
- plastic surgery
- Pulmonology
- Radiation oncology
- Rheumatology
- Thyroid & Endocrine Surgery
- Urology
- Vascular Surgery
- KIMA News
- KIMA Live
- Community
KIMA NEWS
A team of Korean researchers has developed a groundbreaking technology that reduces the time required for sepsis testing from about three days to half a day, marking a world first.
On the 25th, a joint research team from Seoul National University Hospital (SNUH) and Seoul National University announced the publication of their clinical trial results for 'uRAST,' an ultra-rapid antimicrobial susceptibility test, in the international journal 'Nature.' The team includes Professor Park Wan-beom from the Division of Infectious Diseases, Professor Kim Taek-soo from the Department of Laboratory Medicine, Professor Kim In-ho from the Division of Hematology and Medical Oncology at SNUH, and Professor Kwon Sung-hoon from the Department of Electrical and Computer Engineering at Seoul National University, in collaboration with QuantaMatrix Co., Ltd.
Sepsis, a systemic inflammatory response caused by pathogen infection, results in the death of 2-5 out of 10 patients. With the mortality rate increasing by about 9% every hour, rapid treatment is crucial. However, the lengthy process of antimicrobial susceptibility testing to select the appropriate antimicrobial for patients has made timely treatment challenging.
The newly developed 'uRAST' has reduced testing time by an average of 48 hours compared to existing methods. This includes the pre-culture stage of pathogens and the actual antimicrobial susceptibility testing time.
uRAST directly isolates pathogens from blood using synthetic nanoparticles, eliminating the blood culture stage that could take up to 7 days. With a rapid culture stage, the necessary pathogens for testing can be secured within 6 hours.
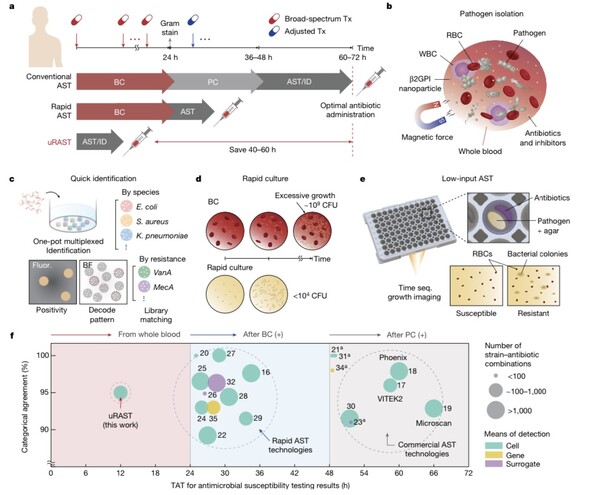
The time required for pathogen identification and antimicrobial susceptibility testing after culture has also been reduced to 6 hours, down from the previous minimum of 24 hours. This result was achieved by incorporating QuantaMatrix's rapid pathogen identification (QmapID) and rapid antibiotic susceptibility test (dRAST). In a clinical trial involving 190 patients suspected of sepsis infection, uRAST completed all testing stages within 13 hours using only 10mL of whole blood. This is about 48 hours faster on average than conventional tests. The research team emphasized that "this is currently the fastest testing technology in the world."
While significantly reducing testing time, the accuracy remained comparable to existing methods. Compared to standard testing methods, uRAST's susceptibility test CA (Categorical Accuracy) was 94.4%, meeting FDA standards. The research team explained, "This means the accuracy is high, similar to standard testing methods."
Professor Park stressed, "Some patients die because they cannot receive optimal antimicrobials in time due to the time required for antimicrobial susceptibility testing. uRAST will increase patient survival rates and revolutionize sepsis treatment."
Professor Kim Taek-soo stated, "uRAST integrates all necessary diagnostic testing processes shortly after blood collection. It's a breakthrough in sepsis diagnosis. We hope that uRAST will be used as a new medical technology to quickly identify pathogen types and find effective antimicrobials, improving the prognosis of sepsis patients."
Inquiry