RELATED Live.
- About us
- KIMA Members
-
KIMA Doctors
- All
- Anesthesiology
- artificial joint center
- Breast and Endocrine Surgery
- Breast cancer and thyroid cancer center
- Breast Surgery
- Cardiology
- Cardiothoracic Surgery
- Cerebrovascular Center
- Colorectal Surgery
- dental and maxillofacial surgery
- Dermatology
- Endocrinology
- Gastroenterology
- General Surgery
- Genito-Urology
- Hematology
- Hemato-oncology
- Infection Center
- Internal Medicine
- International Healthcare Center
- Korean Medicine
- liver center/Pancreas and billiary tract center
- Liver Transplantation
- Neurology
- Neurosurgery
- Obstetrics & Gynecology
- Ophthalmology
- Orthopedic
- Otorhinolaryngology
- Pediatric & Juvenile
- Pediatric Allergy and Respiratory Diseases
- Pediatric Gastroenterology
- Pediatric Neurology
- Pediatrics
- Physical Medicine & Rehabilitation
- Plastic & Reconstructive Surgery
- plastic surgery
- Pulmonology
- Radiation oncology
- Rheumatology
- Thyroid & Endocrine Surgery
- Urology
- Vascular Surgery
- KIMA News
- KIMA Live
- Community
KIMA NEWS
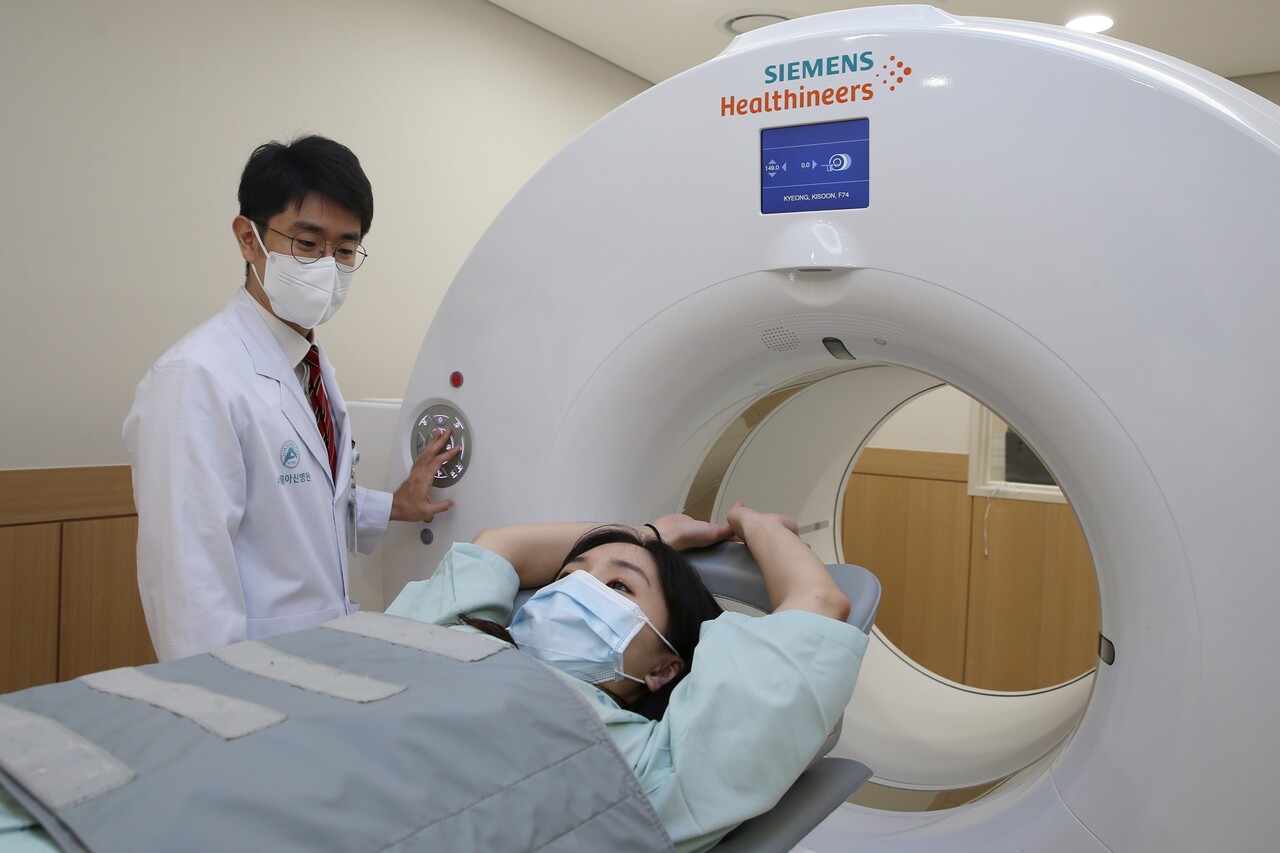
‘18F-FES PET Test’ in NCCN Guidelines
A breast cancer imaging diagnostic technique developed by the medical team at Asan Medical Center(AMC) has been indexed as a global standard test. Even in cases where a tissue test is challenging for breast cancer patients, estrogen receptors can be diagnosed in approximately 15 minutes through imaging alone. The treatment for breast cancer varies depending on the presence of these estrogen receptors.
Seoul Asan Medical Center announced on the 14th that the 18F-FES (fluoroestradiol) positron emission tomography (PET) test, developed by a research team including Professors Moon Dae-hyuk and Han Sang-won of the Nuclear Medicine Department at the AMC’s breast cancer center, Professor Kim Sung-bae from oncology, and Professor Lee Jong-won from breast surgery, has been included in the guidelines of the National Comprehensive Cancer Network (NCCN), an esteemed organization that sets the global standard for cancer treatment.
In Korea, estrogen receptor-positive breast cancer, which accounts for 70% of breast cancer patients, requires hormone therapy as the cancer cells grow due to hormones. If the breast cancer recurs or metastasizes, the treatment is determined based on the estrogen receptor diagnosis. Therefore, additional tissue tests are necessary, but these tests can be complex depending on the area.
The 18F-FES PET test developed by Seoul Asan Medical Center's medical team can scan lesions that have spread throughout the body in about 15 minutes. The Breast Cancer Center spearheaded the development and clinical application of the 18F-FES PET test, verifying its safety and effectiveness. The research findings were also adopted as a primary basis when the American Society of Nuclear Medicine and the Korean Society of Nuclear Medicine announced the appropriate use criteria for the 18F-FES PET test.
Professor Moon commented, “I am pleased that the 18F-FES PET test, whose safety and effectiveness have been proven through research in Korea, has been recommended in the NCCN guidelines. Patients with recurrent or metastasized breast cancer, for whom tissue testing was difficult or impossible, can now be diagnosed more safely and accurately for the presence of estrogen receptors and receive the most suitable treatment.”
He added, “We will continue to lead global research to increase cancer patients’ survival rates and provide personalized treatment.”
Kim Sung-bae, Director of the Breast Cancer Center, stated, “With the advancement of breast cancer diagnostic techniques like the 18F-FES PET test, along with developments in chemotherapy, hormone therapy, and radiation treatment, we are aiming for a five-year survival rate of 95% for breast cancer patients. Especially for estrogen receptor-positive breast cancers, which have a relatively higher risk of late recurrence and for which the receptors can change, if patients remain hopeful and actively engage in treatment, they can achieve favorable outcomes.”
Inquiry